ChE Process Design and Optimization
Course Description
The Chemical Engineering Program’s capstone design sequence includes Process Design and Optimization I and II (CHE 433 and 434, respectively). In these courses, students integrate content from earlier courses for complex, open-ended design assignments. As the students progress through ChE 433, their assignments require increasingly more effort, initiative, knowledge and individual responsibility. In CHE 434, students design an entire commercial-scale chemical plant and use detailed economic analyses to optimize the plant’s profitability. For over 50 successive years, MSU’s CHE 434 students have worked intensively one to two months to develop a solution to the annual American Institute of Chemical Engineering (AIChE) Student Design Competition problem. MSU’s Chemical Engineering Program uses these realistic, industry-based problems to enhance chemical engineering students’ capstone design experience for three reasons: 1) the AIChE problems provide real-world, open-ended design experiences typical of those students are likely to face after graduation; 2) they require students to do self-directed, active learning, including project- specific research, to solve the problem; and 3) they serve as a national benchmark for MSU’s chemical engineering students to demonstrate excellence in their professional skills.
As the Chemical Engineering program’s contribution to Design Day, several CHE 434 students present posters describing their solutions to this year’s AIChE Student Design Competition problem. Names and pictures of the presenters are provided at the end of this article.
National Awards in 2020 Design Competition
Since 1968, MSU has had the best record nationally for awards in the AIChE Student Design Competition. In the 2020-2021 competition, four MSU students won national awards. In the individual category, Evan Litch won first place, receiving the A. McLaren White Award for best overall design. Austin Alexander won first place in the Best Applications of the Principles of Chemical Safety, receiving the Walter Howard Design Award in the individual category. Alexander also took second place in the best overall design category, receiving the A.E. Marshall Award. Additionally, two MSU students were awarded with the top honor in this year’s team category. Austin Jenner and Ian Scheper won the Jack Wehman Design Award in the Best Applications of the Principles of Chemical Process Safety team category. All four students graduated in 2021 with degrees in chemical engineering.
Gas-to-Liquid-Fuel Modular Manufacturing Plant
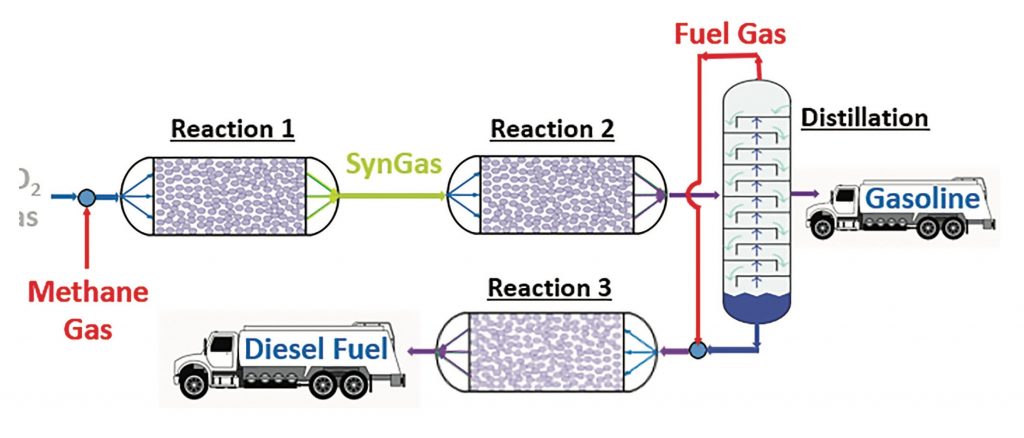
This year’s AIChE Student Design Competition problem is to design relatively compact, “modular” chemical plants to produce liquid transportation fuels, such as synthetic gasoline and diesel fuels, from the gases methane (CH4) and carbon dioxide (CO2). The novel modular approach is optimal for recently discovered natural gas deposits in remote locations, where it would not be cost-effective to build full-scale petrochemical processing plants. At these remote sites, the energy contained in the natural gas (predominantly methane) would be concentrated in the form of liquid transportation fuels and then transported to a central distribution facility. Simultaneously, the greenhouse gas CO2 would be captured in a useful product, rather than being released into the atmosphere.
The process flowsheet for this problem is shown in Figure 1. First, the methane and carbon dioxide gases are combined and converted in Reaction 1 into a synthesis gas (SynGas), which is a mixture of carbon monoxide and hydrogen gases. Then, the SynGas is converted in Reaction 2 into a mixture of gas and liquid products. Next, the mixture is separated by distillation into a fuel gas stream, a synthetic gasoline product, and a heavier liquid stream. Finally, the heavier liquid and fuel gas streams are combined and converted in Reaction 3 into a synthetic diesel fuel product.
While the chemical reactions involved in this design challenge problem are not novel, the increased emphasis placed on minimizing the modular plants’ energy consumption and environmental impact is. In addition, an increased emphasis is placed on having students base their design decisions on a broad range of public health, safety, and welfare concerns, as well as make informed judgments considering the impact of their engineering solutions in global, economic, environmental, and societal contexts.
Students submit their solutions to the AIChE Student Design Competition problem as reports up to 150 pages long. These reports include details of the manufacturing plant’s equipment, operating conditions, personnel needs, capital investment, and a complete economic analysis that gives the expected discounted-cash-flow rate of return on the company’s investment. The reports are graded based on both their technical quality and their communication effectiveness. Because design reports in industry must be understandable by high-level decision makers having various academic backgrounds, the reports must communicate effectively with a wide range of audiences.
Gas-to-Liquid- Fuel Process Flowsheet for 2021-2022 AIChE Student Design Competition Problem
Student Poster Presenters on Design Day
The CHE 434 students below are presenting a lay-level poster of their design solutions to the AIChE Design Competition problem and discussing with visitors the advantages of careers in chemical engineering.
Students Presenting an Individual Solution to the AIChE Team Competition Problem
Students presenting an Individual Solution to the AIChE Team Competition Problem include Auden Chase (pictured) and Nishan Rankothge (picture unavailable).
Students Presenting a Two- Person Solution to the AIChE Team Competition Problem
Students presenting a Two-Person Team Solution to the AIChE Team Competition Problem include Shay and Emma Smith (pictured) and Mercy Crocker and Carson Malhado (picture unavailable).